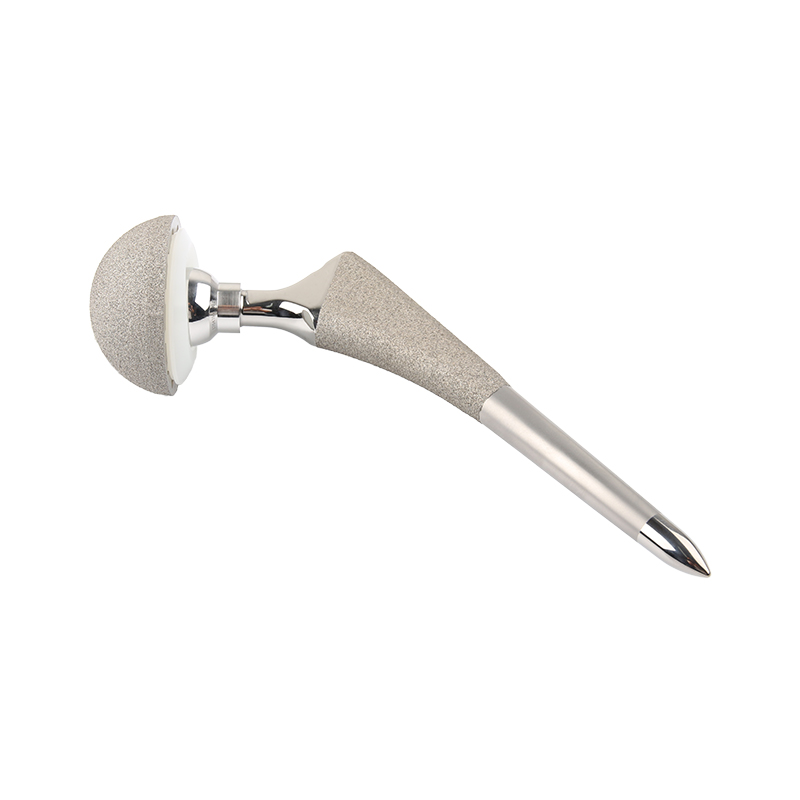
Artificial Hip Joint FDH Femoral Head
Indications
Total Hip Arthroplasty (THA) is a surgical procedure that aims to improve patient mobility and alleviate pain by replacing a damaged hip joint with artificial components. This procedure is only recommended for patients who have sufficient healthy bone to support the implants. Generally, THA is performed on individuals who are suffering from severe pain and/or disability caused by conditions such as osteoarthritis, traumatic arthritis, rheumatoid arthritis, congenital hip dysplasia, avascular necrosis of the femoral head, acute traumatic fractures of the femoral head or neck, failed previous hip surgeries, or specific cases of ankylosis.On the other hand, Hemi-Hip Arthroplasty is a surgical option used in cases where there is evidence of a satisfactory natural acetabulum (hip socket) and enough femoral bone to support the femoral stem. This procedure is indicated for various conditions including acute fractures of the femoral head or neck that cannot be effectively treated with internal fixation, fracture dislocation of the hip that cannot be appropriately reduced and treated with internal fixation, avascular necrosis of the femoral head, non-union of femoral neck fractures, certain high subcapital and femoral neck fractures in elderly patients, degenerative arthritis that affects only the femoral head and does not require acetabulum replacement, and specific pathologies involving the femoral head/neck and/or proximal femur that can be adequately addressed with hemi-hip arthroplasty.The choice between Total Hip Arthroplasty and Hemi-Hip Arthroplasty depends on several factors such as the severity and nature of the hip condition, the age and overall health of the patient, as well as the surgeon's expertise and preference. Both procedures have proven to be effective in restoring mobility, reducing pain, and improving the quality of life for patients suffering from various hip joint disorders. It is important for patients to consult with their orthopedic surgeons to determine the most suitable surgical option based on their individual circumstances.
Clinical Application